Enabling Australia’s mRNA therapeutics production and development
We are an Australian contract development and manufacturing organisation (CDMO) specialising in the production of nucleic acid products that support life-enabling work. We support our clients by providing raw materials and scalable manufacturing capacity and help them accelerate innovation in bringing their products where they are needed – benchtop to bedside or field.
Our goal at Southern RNA is to be a world leading, customer-focused, end-to-end supplier of nucleic acid products such as mRNA and DNA. We strive towards this goal daily by developing and deploying our state-of-the-art bioprocessing and chemical process technologies, using proprietary manufacturing and analytical approaches, employing a highly-skilled workforce and partnering with an excellent and extensive network of relationships and collaborations.
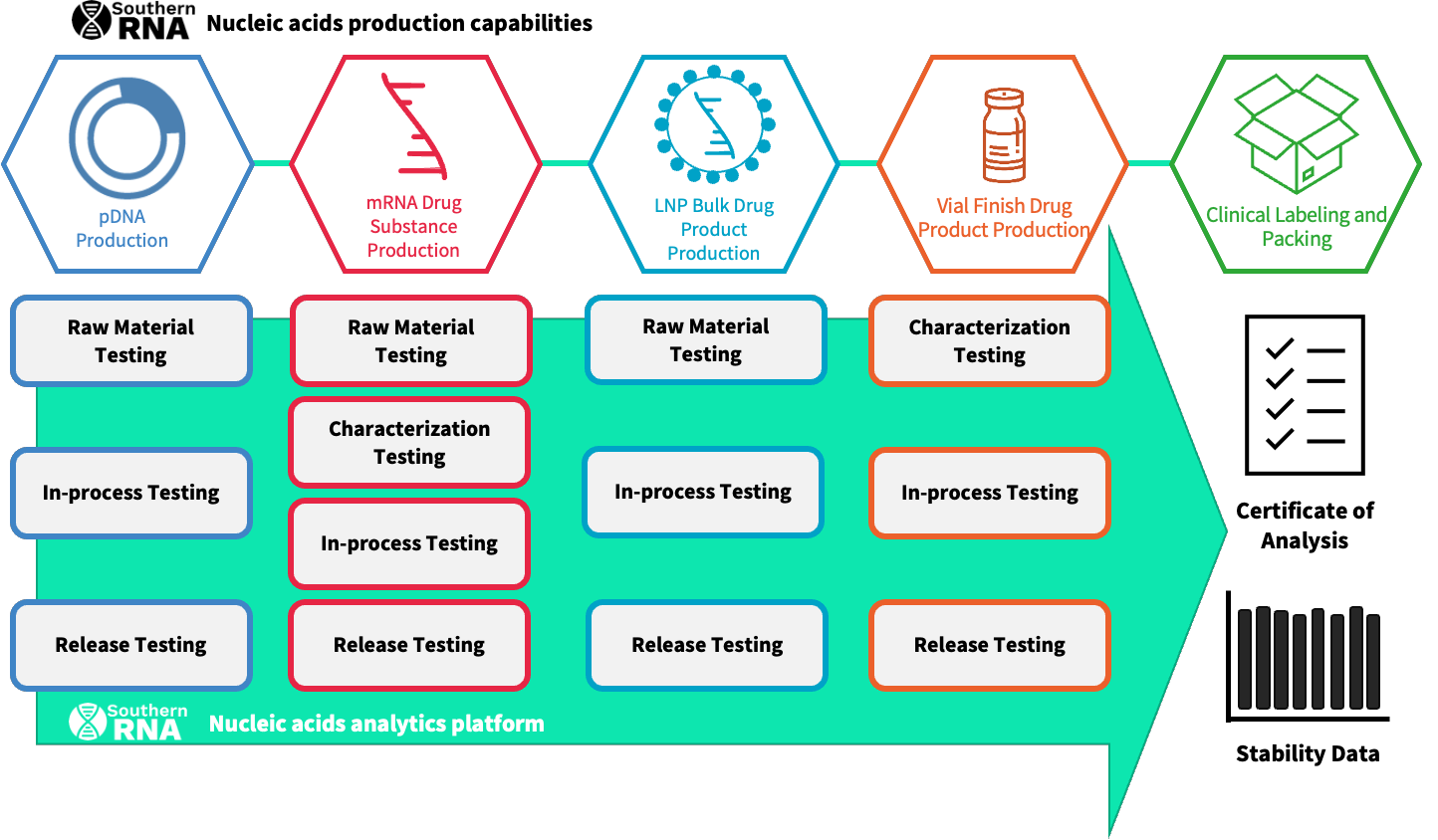
Establishing End-to-End Capability
We are executing our vision to deliver the capabilities needed in Australia to support the clinical development of new therapies through clinical-grade RNA production services.
Our Development Stages
STAGE 1 – Completed
RAW MATERIAL PRODUCTION
STAGE 2 – In progress
cGMP mRNA PRODUCTION
STAGE 3 – Upcoming
FULL SERVICE PROVIDER
OUR COMPETITIVE ADVANTAGE
LOCATION
SRNA is located at proximity to Brisbane, rated as a top 20 livable city in the world with world-class research institutes, and strong connections to global research and academic community.
PLATFORM TECHNOLOGY
Proven proprietary processes, products, and services to support your nucleic acid vaccine and therapeutic programs and state-of-the-art equipment that enable scalable high-quality production.
END-TO-END SUPPORT
Construct design support, development services, best-in-class raw materials, upcoming GMP final products, and regulatory support for you at every stage of your pipeline.
TALENT
World-class pan-disciplinary talent across chemistry, biology, biochemistry, biophysics, supply chain, quality management, business development, and more – all working to support you.
TRADE


